-
 STUDY MATERIALS
STUDY MATERIALS
-
 COURSES
COURSES
-
 MORE
MORE
Back
- JEE ADVANCE
- JEE MAIN
- NEET
- BITSAT
- CBSE BOARD
- UP BOARD
JEE ADVANCE
- CLASS XI - XII
JEE MAIN
- CLASS XI - XII
NEET
- CLASS XI - XII
BITSAT
- CLASS XI - XII
CBSE BOARD
- CLASS IX
- CLASS X
- CLASS XI
- CLASS XII
- CLASS VI
- CLASS VII
- CLASS VIII
CLASS IX
CLASS X
CLASS XI
CLASS XII
- PHYSICS
- CHEMISTRY
- MATHS
- BIOLOGY
- NCERT PHYSICS
- NCERT CHEMISTRY
- NCERT MATHS
- NCERT BIOLOGY
- NCERT PHYSICS (Hindi)
- NCERT CHEMISTRY (Hindi)
- NCERT MATHS (Hindi)
- NCERT BIOLOGY (Hindi)
- NCERT ENGLISH
- NCERT ACCOUNTANCY
- NCERT ECONOMICS
- NCERT BUSINESS STUDIES
- NCERT ACCOUNTANCY (Hindi)
- NCERT ECONOMICS (Hindi)
- NCERT BUSINESS STUDIES (Hindi)
CLASS VI
CLASS VII
CLASS VIII
UP BOARD
- CLASS IX
- CLASS X
- CLASS XI
- CLASS XII
CLASS IX
CLASS X
CLASS XI
Courses
- CLASSROOM COURSES
- ONLINE COURSES
ONLINE COURSES

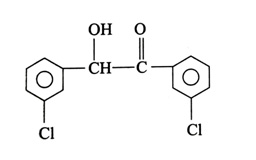
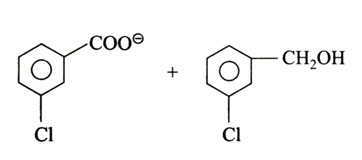
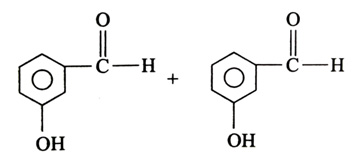
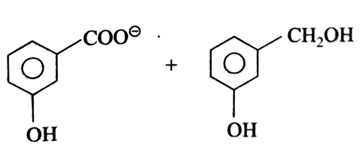
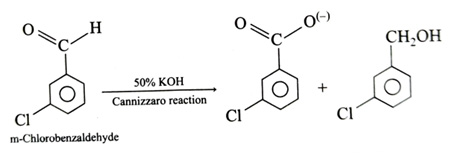
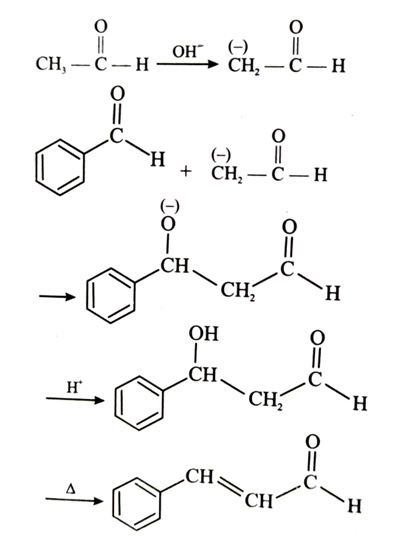
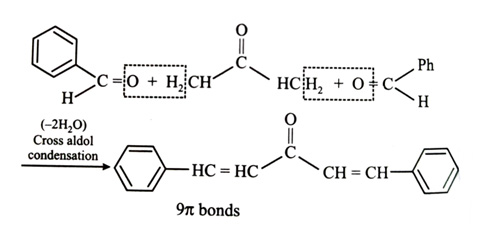
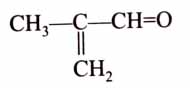
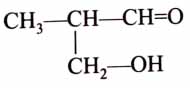
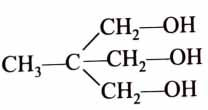
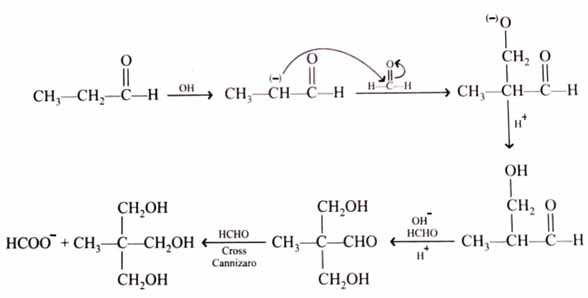
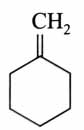
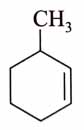
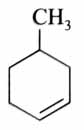
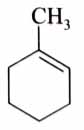
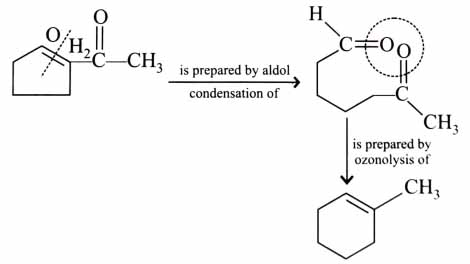
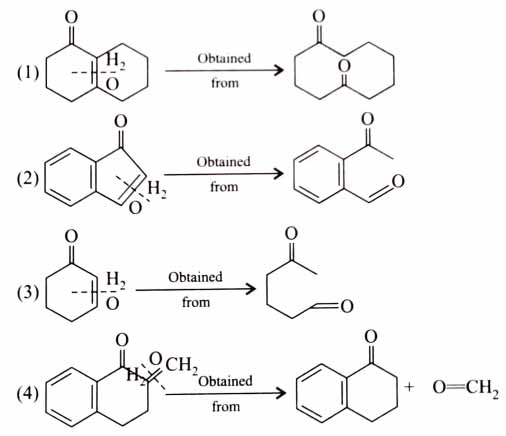
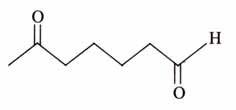
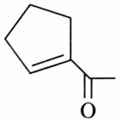
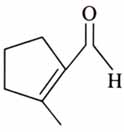
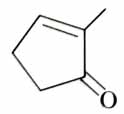
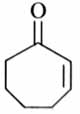
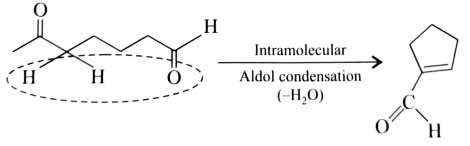
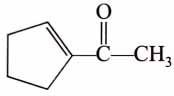 (x). He thought of preparing the molecule using an aldol condensation reaction. He found a few cyclic alkenes in his laboratory. He thought of performing ozonolysis reaction on alkene to produce a dicarbonyl compound followed by aldol reaction to prepare “x”. Predict the suitable alkene that can lead to the formation of “x”. [2025]
(x). He thought of preparing the molecule using an aldol condensation reaction. He found a few cyclic alkenes in his laboratory. He thought of performing ozonolysis reaction on alkene to produce a dicarbonyl compound followed by aldol reaction to prepare “x”. Predict the suitable alkene that can lead to the formation of “x”. [2025]